Are you ready for
sinus relief?
Find help for your Sinusitis.
See if you may be a candidate for advanced treatment for chronic sinusitis.
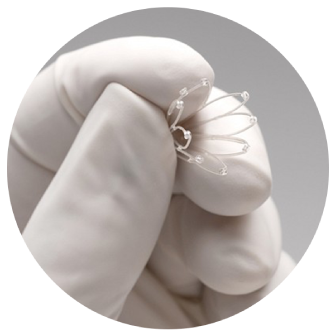
Sinus surgery paired with the PROPEL® sinus stent is clinically proven to provide relief for people suffering from chronic sinus issues.1
1. Han JK, Marple BF, Smith TL et. al., Int. Forum Allergy Rhinol. 2012
The purpose of the site is to help create awareness about sinusitis and treatment options for the disease. Please note that information contained on this site is not medical advice. It should not be used as a substitute for speaking with your physician. Always talk with your physician about diagnosis and treatment information.
The PROPEL sinus implants are intended for use following sinus surgery to maintain the sinus openings and to locally deliver a drug to the sinuses: PROPEL for use in the ethmoid sinus, PROPEL Mini for use in the ethmoid sinus and frontal sinus opening and PROPEL Contour for use in the frontal and maxillary sinus openings. The products are intended for use in patients ≥18 years of age. These products are not intended for people who are allergic to the drug (mometasone furoate) or to certain polymers. Safety and effectiveness of the implant in pregnant or nursing females have not been studied. Risks may include, but are not limited to, pain/pressure, movement of the implant (within or out of the sinus), possible side effects of the drug,infection, and nose bleed. For more information on the risks and benefits of PROPEL sinus implants, please talk to your doctor. The FDA approved labeling can be found at www.IntersectENT.com. Rx only.
INTERSECT ENT and PROPEL are registered trademarks of Intersect ENT, Inc. in the United States and other countries.
MPM 00374 Rev. A
AVAILABLE NOW
Ph: (855) 223-0717

|
|||||||||||||||
|
|
|||||||||||||||